If you want to access the European and/or international market, are looking for a mandatary or a PCVRR or want to access reimbursement for your products on the French market, CEISO can assist you.

Are you a manufacturer outside the European Union planning to sell your medical devices in the European market ?
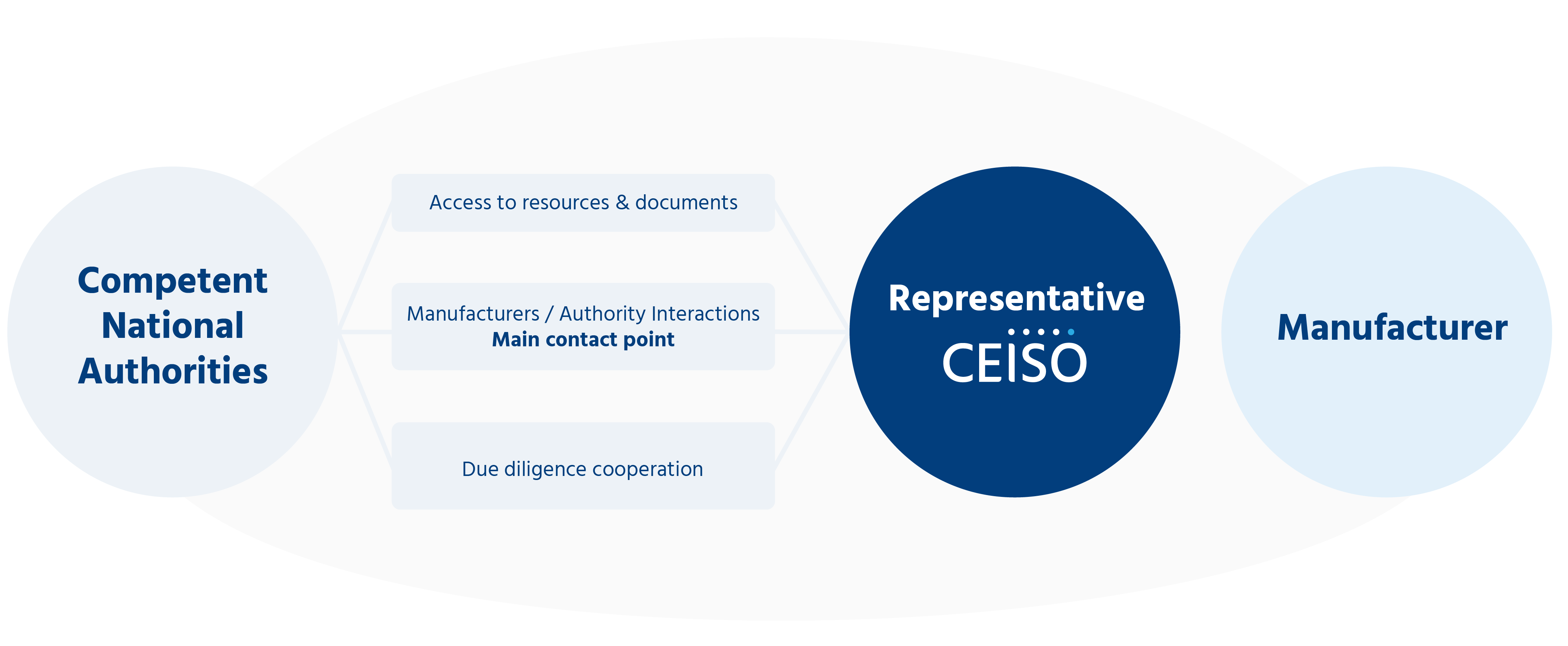
The regulation requires you to use a mandatary.
We are at your disposal as a registered representative on the EUDAMED database under number FR-AR000000342.
Are you located in Europe and plan to sell your devices there?
You must comply with the European regulation for medical devices (2017/745 and 2017/746).

So that your device complies with European safety and performance requirements.
Are you a mandatary or a small business subject to European regulation on medical devices?
You are required to have a Person Responsible for Regulatory Compliance with this regulation (PRRC).
This function can be outsourced.
We provide you with the necessary competent resources.
A mandatary is “any natural or legal person established in the Union who has received and accepted a written mandate from a manufacturer established outside the Union, to act on behalf of the manufacturer for the fulfillment of specified tasks related to the obligations of that manufacturer under this Regulation”.
This required representation allows authorities to be able to contact a responsible person who is based in Europe and designated to act on behalf of a manufacturer located outside Europe, especially in case of emergency.
Do you want to sell your medical devices in China or in the North American market?
We can assist you in your efforts:
CEISO assists you in preparing the file for reimbursement of your products and services.
Any application for registration of your medical device on the list of reimbursable products and services (LPPR) must be submitted to the National Commission for the Evaluation of Medical Devices and Health Technologies (CNEDiMTS) under article L.165-1 of the Social Security Code.
CEISO assists you in preparing your reimbursement application file.
Does your problem concern market access?
Please specify your need via the contact form.
We will get back to you as soon as possible.