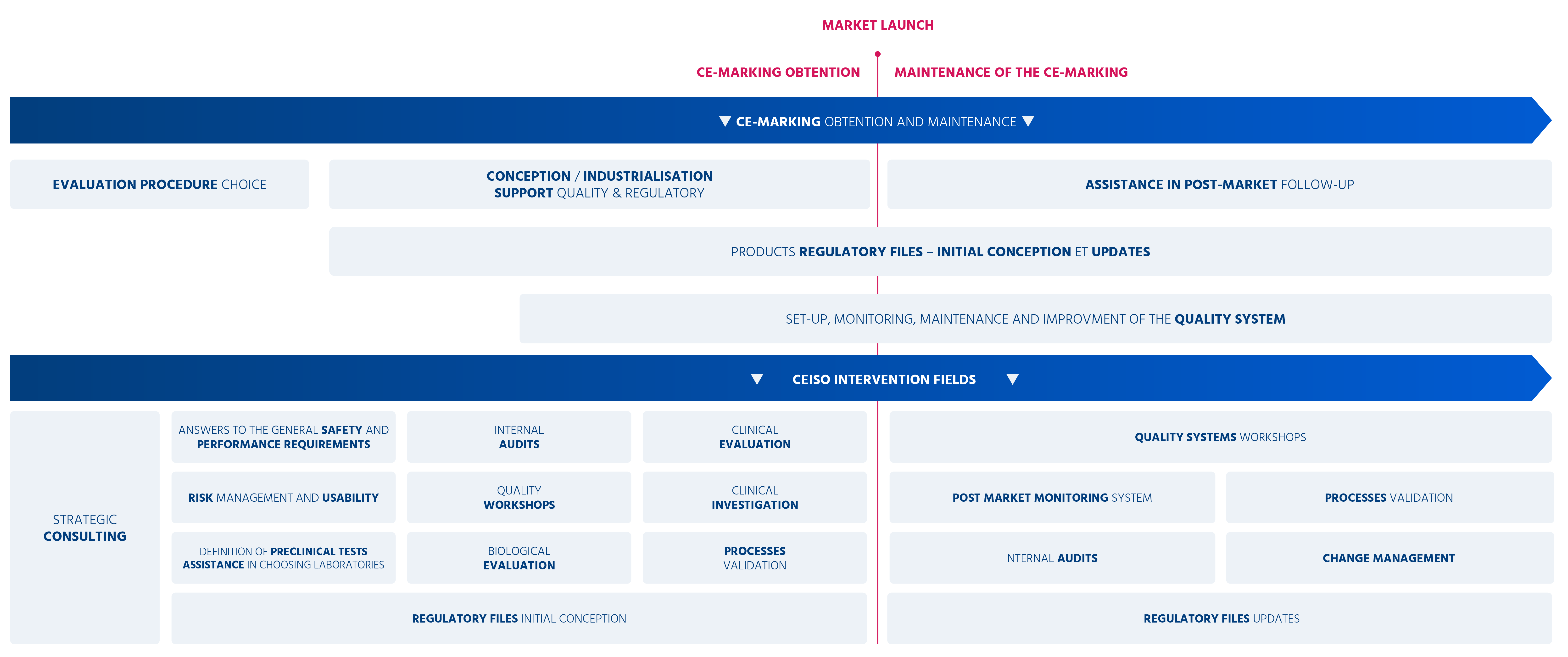
From strategic consulting to post-commercialization follow-up assistance, our team works with you to assist you in bringing your products and services to market successfully.
If you have specific issues or questions regarding regulatory strategy affecting the development of your company or products, we offer our experience to provide advice on the application of regulations …
If you are a start-up and wish to access a variety of markets …
We offer our experience for ad hoc advice on the application of regulations.
You need to implement a Quality Management System, and adapt it in accordance to regulatory constraints and to your objectives?
We can assist you in all stages of your project.
Our services include internal and subcontractor audits as well as preparation for certification audits or inspections by competent authorities.
Our support is tailored to your needs.
For the implementation of a Quality System in accordance with quality reference frameworks in the biomedical sector (ISO 13485, 21 CFR 820, GLP, GMP, etc.).
For the maintenance, evolution and control of your Quality System, adapting it to your strategy and normative and regulatory developments.
Simplifying and optimizing your Quality System to be more agile, adaptable and, therefore, efficient.
If you plan to market a device, you are required to prepare technical files that comply with the requirements of applicable regulations.
Depending on the risk profile of the product, its review by regulatory agencies may be required.
These files must be presented in a clear, organized, unambiguous, and easily consultable way, and must include all the elements required by regulations.
If you want to assess the functioning and effectiveness of your quality system objectively, and if you lack the resources to carry out an internal audit, CEISO puts its team of auditors at your service, to carry out your audits.
In addition to the audit findings, we offer improvement suggestions throughout the process.
As part of your obligations to monitor your Quality System (QMS).
With the objective of preparing for your certification audits or inspections by health authorities.
Audits prepared in advance with you to ensure compliance with your providers' contractual obligations.
As part of operational problem-solving, validation of your processes, company takeover, supplier/subcontractor selection.
Assistance services during certification audits, on-site inspections or summons by health authorities.
If you want to place a device on the European market, you must comply with applicable regulations.
Our multidisciplinary team is at your side to meet all your obligations.
We assist you in demonstrating the safety and performance of your device, in particular through:
Regulatory and normative monitoring is a requirement. It allows for the anticipation of regulations and normative texts that may have an influence on your products, activities or business strategy.
In the face of frequent regulatory changes and the growing complexity of the texts, benefit from effective regulatory and normative monitoring service, all the while mobilizing a minimum of internal resources.
Is your device in direct or indirect contact with the body? You need to be aware of toxicological and biological risks.
Our services are carried out with the expertise of toxicologists whose skills are recognized at the European level.
Upon request, we can assist you with preparing for potential testing protocols and identifying suitable laboratories
For each marketed medical device, the manufacturer must design, establish, document, apply, maintain and update a post-market surveillance and clinical surveillance system.
If you are having trouble implementing these requirements, we can provide training and support you in the development and application of this surveillance.
Does your problem concern Quality and/or Regulations?
We invite you to specify your need via the contact form.
We will get back to you as soon as possible.