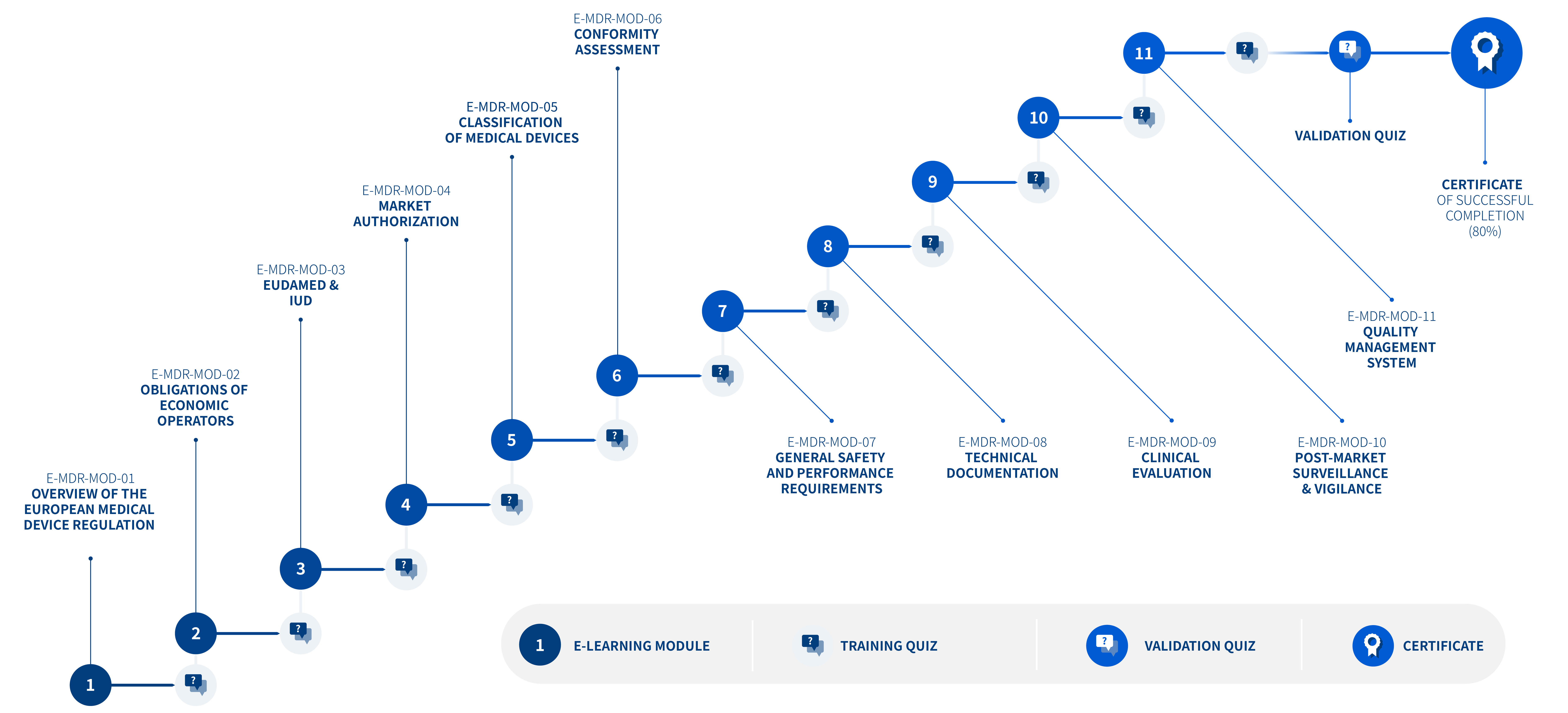
E-MDR-MOD-01 – Overview of the European Medical Device Regulation
REF. E-MDR-MOD-01 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Overview of the European Medical Device Regulation module
CEISO ACADEMY: our turnkey E-learning service to support you in understanding and implementing the European Regulation on Medical Devices (EU) 2017/745, which came into effect in 2021.


CEISO ACADEMY aims to facilitate the self-paced skill-building of manufacturers, authorized representatives, importers, and distributors of medical devices. This is a necessary prerequisite for greater efficiency from product design to market entry and throughout their life cycle management.
REF. E-MDR-MOD-01 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Overview of the European Medical Device Regulation module
REF. E-MDR-MOD-02 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Obligations of Economic Operators module presents the parties
REF. E-MDR-MOD-03 – E-LEARNING EUROPEAN REGULATIONS Regulation (EU) 2017/745 COURSE DESCRIPTION The EUDAMED & UDI module presents the EUDAMED database
REF. E-MDR-MOD-04 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Market Authorization module presents the principles and steps
REF. E-MDR-MOD-05 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Classification of Medical Devices (MD) module presents the
REF. E-MDR-MOD-06 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Conformity Assessment module presents the procedures to assess
REF. E-MDR-MOD-07 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The General Safety and Performance Requirements (GSPR) module presents
REF. E-MDR-MOD-08 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Technical Documentation module presents the content of the
REF. E-MDR-MOD-09 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Clinical Evaluation module presents the clinical evaluation methodology
REF. E-MDR-MOD-10 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Post-Market Surveillance & Vigilance module describes the purpose
REF. E-MDR-MOD-11 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Quality Management System module presents the manufacturer’s obligations
REF. E-MDR-F-01 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The MANUFACTURERS’ package is composed of 11 modules and
REF. E-MDR-F-02 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The DISTRIBUTORS’ package is composed of 7 modules and
REF. E-MDR-F-03 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The IMPORTERS’ package is composed of 7 modules and
REF. E-MDR-F-04 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The AUTHORIZED REPRESENTATIVES’ package is composed of 9 modules
REF. E-MDR-F-05 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The REGULATORY AFFAIRS package is composed of 11 modules
REF. E-MDR-F-06 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The RESEARCH & DEVELOPMENT package is composed of 6
REF. E-MDR-F-07 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The QUALITY ASSURANCE package is composed of 7 modules
REF. E-MDR-F-08 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The CLINICAL DEPARTMENTS’ package is composed of 5 modules
REF. E-MDR-F-09 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The Production/Purchasing package is composed of 5 modules and
REF. E-MDR-F-10 – E-LEARNING EUROPEAN REGULATIONS REGULATION (EU) 2017/745 COURSE DESCRIPTION The SALES DEPARTMENTS’ package is composed of 6 modules
Your question concerns our e-learning courses? We invite you to specify your needs via the contact form.
We will get back to you as soon as possible.