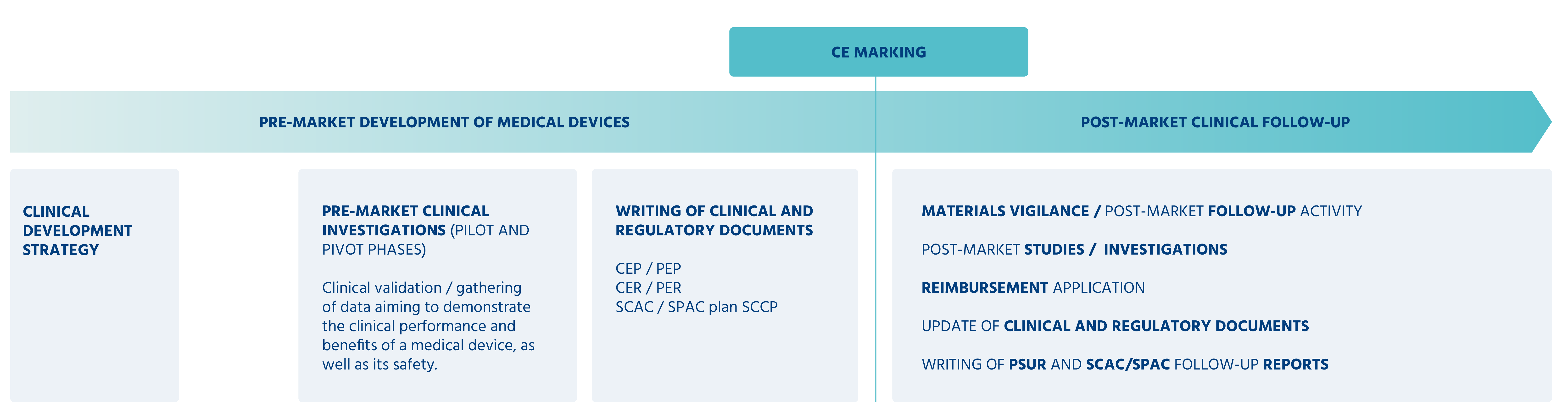
It is dedicated to clinical trials (C.R.O.) and clinical-regulatory support (development of clinical evaluation and post-market clinical follow-up documents) of Medical Devices (MDs and IVDs).
MediaClin is approved for Research Tax Credit.

Do you want to do a pre- or post-market clinical trial of one of your medical devices?
Do you want to design/update the clinical-regulatory documents of a medical device?
Pre/post-market strategy, compliance, implementation, MD requirements.
Have your clinical trial verified for compliance with applicable regulations and norms.
We can support you in the development and maintenance of your team's skills.
With 20 years of experience, MediaClin provides its clients with expertise in different application areas such as Rheumatology, Ophthalmology, Cardiology, Traumatology, Pneumology, Gastroenterology, Osteology, Dermatology, E-Health, as well as on medical devices including software as Medical Devices and In Vitro Diagnostic Medical Devices.
Expertise in ISO 13485, ISO 14155 and ICH-GCP, ISO 14971, MDR 2017/745, IVDR 2017/746.
Caval vein filter, vascular stent, angioplasty balloon, holter…
Gastric probe, companion test, self-diagnostic…
Esophageal vibration generator, respirators, respiratory masks…
Connected Medical Device in endocrinology, Telemonitoring Medical Device…
Anal fissure gel, cryotherapy medical device, psoriasis treatment…
Magnetic field generator…
Orthoses, injectable gel…
Orthodontic retainer, anti-xerostomia lozenges…
Intraocular lenses, ocular movement analysis software…
Duodenal sleeve, dietary supplements, intragastric balloon…
Orthoses, injectable gel…
Articular implantable gel, maxillofacial osteosynthesis plates and screws…